Background
The diagnosis of and distinction between acute myeloid leukemia (AML) and high-risk myelodysplastic syndrome (MDS) have long been based on blast counts, with the presence of ≥20% myeloblasts in marrow or peripheral blood required to define AML in World Health Organization classification. Until 2001, blast counts greater than 30% had been used to diagnose AML by the original French-American-British classification system. However, the 2022 International Consensus Classification proposed a new combined category of MDS/AML with 10-19% blasts. Here we report the real-world treatment pattern and outcomes of AML and high-risk MDS patients at an academic institution in the United States, with specific attention to the blast burden at initial disease diagnosis to validate accuracy and limitation of this new proposed change.
Methods
This is a retrospective study conducted at UCLA Health encompassing all diagnoses of AML and MDS made at academic campus and community practices. We evaluated adult patients with a diagnosis with either AML or MDS from 1/1/2016-7/31/2022. For patients with more than one bone marrow biopsy on record, we recognized the date of diagnosis of MDS or AML with a blast burden ≥ 10% by immunohistochemistry or flow cytometry as the first bone marrow biopsy. In light of the changing diagnostic definitions, we analyzed patients by cohorts of MDS/AML with 10-19% blasts, AML low-blast with 20-29% blasts, and AML high-blast ≥ 30% blasts. Survival analysis by blast count at diagnosis was calculated by Kaplan-Meier estimates. A Multivariable Cox proportional hazard model was used to model survival outcomes by blast cohort, ELN risk 2022, response to first treatment, and receipt of allogeneic hematopoietic cell transplantation (HCT).
Results
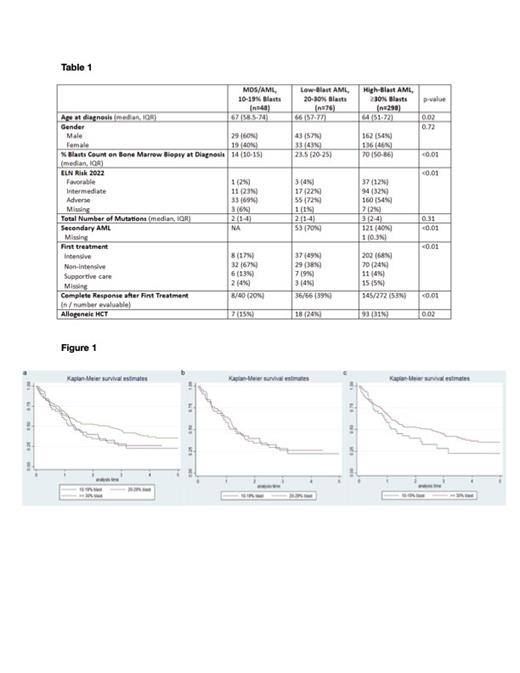
There were 422 patients at UCLA Health with a diagnosis of AML or MDS included in our analysis with blast burden ≥ 10%. Patient demographics are shown in Table 1. Median age at diagnosis for MDS/AML, AML low-blast and AML high-blast cohorts is 67, 66 and 64 respectively (p=0.02). Thirty two (66.7%) patients in MDS/AML cohort received non-intensive first line treatment, whereas 202 (67.8%) AML high-blast patients received intensive first treatment. For AML low-blast patients, 37 (48.7%) received intensive treatment, and 29 (38.2%) received non-intensive treatment. More patients with AML (either low- or high-blast) underwent allogeneic HCT compared to MDS/AML patients. By Kaplain Meier analysis (KM) using blast counts as the sole predictor (Figure 1), no significant difference in OS when following patients for up to five years from diagnosis (p=0.0985) was seen. The p-value for two-way KM comparisons for MDS/AML vs. AML low-blast is 0.9682, and for AML low-blast vs. AML high-blast is 0.112. By multivariable analysis, ELN Risk 2022 (p<.0001), allogeneic HCT (p<.0001), and Complete Response to initial treatment (p<.0001) were predictive of OS, but blast percentages were not (p=0.56).
Conclusion
In our study, we found similar survival outcomes in high-risk MDS and AML patients regardless of initial blast counts at diagnosis. Though not statistically significant, MDS/AML and AML with low-blast burden appear to share more similar outcomes. This could be due to increased secondary AML seen in the low-blast cohort, and implies potentially different disease biology between high blast burden AML and AML with dysplastic changes. With this observation, we emphasize caution on simply using ICC 2022 to apply traditional AML therapy to the new MDS/AML category. Both WHO and ICC classification in 2022 reflects the increasing potential of molecular profiling to subtype these diseases, which will be the future direction. Rather than moving the blast count cutoff between AML and MDS, it is more critical to emphasize inclusion of high-risk MDS patients on all AML clinical trials and perhaps the converse for low blast burden AML with dysplastic changes, which might allow better understanding of response to therapy and disease biology.
Disclosures
Shang:ReviR Therapeutics: Consultancy. Oliai:Pfizer: Research Funding; Jazz Pharmaceuticals: Research Funding; Arog: Research Funding; Seagen: Research Funding; Novartis: Research Funding; Orca Bio: Research Funding. Schiller:Bristol Myers Squibb: Current equity holder in publicly-traded company, Research Funding, Speakers Bureau; Amgen: Current equity holder in publicly-traded company, Research Funding; Johnson & Johnson: Current equity holder in publicly-traded company; Ono Pharmaceutical: Consultancy; Agios: Consultancy; Celgene: Consultancy, Research Funding; Incyte: Consultancy, Research Funding, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding, Speakers Bureau; Astellas Pharma: Consultancy, Research Funding, Speakers Bureau; Kite: Research Funding, Speakers Bureau; Stemline Therapeutics: Speakers Bureau; Sanofi: Research Funding, Speakers Bureau; Karyopharm Therapeutics: Research Funding, Speakers Bureau; Actinium Pharmaceuticals: Research Funding; Actuate Therapeutics: Research Funding; Arog: Research Funding; Celator: Research Funding; Constellation Pharmaceuticals: Research Funding; Daiichi Sankyo: Research Funding; Deciphera: Research Funding; Delta-Fly Pharma: Research Funding; FORMA Therapeutics: Research Funding; Fujifilm: Research Funding; Gamida Cell: Research Funding; Genentech/Roche: Research Funding; Geron: Research Funding; Mateon Therapeutics: Research Funding; Onconova Therapeutics: Research Funding; Pfizer: Research Funding; Precog: Research Funding; REGiMMUNE: Research Funding; Samus Therapeutics: Research Funding; Sangamo Bioscience: Research Funding; Sellas Life Sciences: Research Funding; Stemline Therapeutics: Research Funding; Takeda: Research Funding; Tolero Pharmaceuticals: Research Funding; Trovagene: Research Funding; Agios: Research Funding; ElevateBio: Research Funding; Ono Pharmaceutical: Research Funding; AVM Biotechnology: Research Funding; Syros Pharmaceuticals: Research Funding; Kronos Bio: Research Funding. Chai-Ho:AbbVie: Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Gilead: Research Funding; Servier: Membership on an entity's Board of Directors or advisory committees; Shattuck Labs: Research Funding; Sun Pharma: Research Funding; Syros: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal